The lawsuit was filed by Jose O., a man from Pennsylvania who was injured by the G2® Inferior Vena Cava Filter (“IVC Filter”) manufactured by C.R. Bard and Bard Peripheral Vascular, Inc.
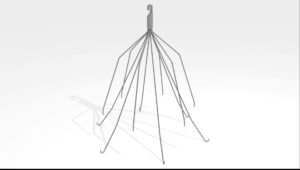
C.R. Bard G2® Inferior Vena Cava Filter
The IVC Filter was surgically implanted in his vein for the purpose of preventing a pulmonary embolism (blood clots in the lungs) on May 21, 2006.
C.R. Bard withdrew the G2 IVC Filter from the market and replaced it with newer models. Several studies have linked the G2 IVC Filter with high rates of fracture and embolization, including a study in 2010 that found 12% of G2 IVC Filters fractured.
Other studies have found up to a 40% risk of fracture associated with the G2 IVC Filter, with the risk increasing the longer it remains implanted in the patient’s inferior vena cava.
The lawsuit accuses C.R. Bard of manufacturing a defective device, failing to warn about side effects, designing a defective device, negligence, negligent misrepresentation, breach of implied and express warranty, and fraudulent misrepresentation.
The lawsuit was filed on December 7, 2017 in the U.S. District Court for the District of Arizona — Case 2:17-cv-04548-DGC
It will be centralized with over 2,000 other IVC filter lawsuits that have already been filed against C.R. Bard in a consolidated federal litigation in Arizona — Multi-District Litigation (MDL No. 2641) — In Re: Bard IVC Filters Products Liability Litigation.
The plaintiff is represented by Ben C. Martin of The Law Offices of Ben C. Martin in Dallas, Texas. He a trial attorney who serves on the plaintiffs’ steering committee of the Bard IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
